Juniper Publishers - Osteoporosis and Osteopenia in Patients with Osteoarthritis
Orthopedics and Rheumatology Open Access Journal
Abstract
Both osteoporosis and osteoarthritis are recognized as age related skeletal disorders, but it is commonly held that these diagnoses do not often occur together in the same patient. Recent studies of bone mineral density scores in subjects with osteoarthritis indicate that osteoporosis occurs worldwide in 21-32% of patients with advanced osteoarthritis. Advanced age and low body-mass-index are major discriminating factors for osteoporosis in osteoarthritis. Because of clinical implications of osteoporosis in patients being managed for osteoarthritis, it is important to identify and refer for osteoporosis evaluation those patients at risk.
Osteoarthritis
Osteoarthritis (OA) most frequently involves the hands, knees, hips, and spine; it is diagnosed on the basis of joint pain and radiographic evidence of non-uniform narrowing of a joint space, osteophytes, and subchondral osteosclerosis and cysts [1,2]. There are three etiological classifications; Type I is genetic, Type II is estrogen-dependent, and Type III is aging-related [3] (Table 1). The generalized pillars of aging that affect other tissues also pertain to cartilage [4]. These include inflammation, oxidative stress, molecular damage, proteostasis, autophagy, and stem cell depletion. Recent data indicate that factors produced by excessive adipose tissue, such as leptin, may play direct roles in pathogenesis and/or progression of OA [5].
The pathophysiology of OA may involve both articular cartilage and subchondral bone. Articular cartilage is avascular, alymphatic, and aneural, with a single cell type, the chondrocyte, capable of both anabolic and catabolic activities [6,7]. Articular cartilage is 70% water and 30% collagen, other noncollagenous proteins, and glycosaminoglycans. Its ultrastructure is critical to its function. The collagen is Type 2, and the collagen fibrils are located in a parallel array at the surface (lamina splendens) and ultrastructural studies reveal that the subsurface collagen fibrils form arcades (arcades of Benninghoff) between the lamina splendens and the subchondral bone. The negatively-charged glycosaminoglycans imbibe water to give an ideal cushion to absorb load. The chondrocytes are arrayed throughout the matrix and receive nutrients that percolate from the synovium through the matrix. This requires a certain pressure on the articular surface, roughly 20-25 kg/cm2. Insufficient load to the cartilage will prevent the nutrients from entering the matrix. Once the upper limit of load is exceeded, the cartilage begins to break down. The chondrocytes attempt to repair, but cannot overcome the disintegration of matrix architecture. The classical understanding of OA is as a problem of wear-and-tear, but this is likely an oversimplification [2,4,5].
There is growing evidence that the subchondral bone may intitiate OA [8]. Radin and Rose proposed that subchondral bone stiffening in OA may result from healing of trabecular microfractures due to joint overloading or misalignment; such an alteration in the biomechanical environment could produce damage in the overlying cartilage [9]. Subchondral thickening and confluence of trabeculae are exceedingly focal, occurring in regions with the thinnest overlying cartilage [10]. Formation of osteophytes, subchondral osteosclerosis, and evidence of increased uptake of radioactive strontium [11] suggest increased bone formation in OA. Studies comparing subchondral bone specimens from OA and control, non-OA subjects showed abnormalities in OA osteoblasts, including decreased responses to PTH and PGE2 and increased urokinase plasminogen activator, alkaline phosphatase activity, osteocalcin, and IGF-I release [12]. A detailed microCT and histological study of subchondral bone from women requiring knee arthroplasty revealed two patient subtypes; subchondral trabecular bone volume doubled under regions with complete loss of cartilage in the majority of specimens [13]. Five of 20, however, were non-sclerotic even with extreme loss of cartilage. The non-sclerotic group showed greater vascular penetration and a greater osteoclastto- osteoblast ratio. These histopathological differences in subchondral bone suggest important metabolic differences that need to be better understood, given the possibility of different therapeutic approaches.
Osteoporosis and Osteopenia
OP is called “a silent disease” because it may not be recognized until a fracture occurs upon minimal trauma, such as a fall from a standing height. OP fractures are also termed fragility or low-energy fractures. A useful distinction was made between Type I (post-menopausal) and Type II (senile) OP, the former being associated with fractures in women within 20 years after menopause and the later occurring in men and women 70 years of age or older [14]. Type I OP is characterized by fractures at sites rich in cancellous bone, such as vertebrae (crush type fractures), wrist, and ankle. Type II OP entails hip and wedge-type vertebral fractures, although fractures of the proximal humerus, proximal tibia, and pelvis are common.
Bone mass decreases with menopause and age due to an excess of bone resorption relative to formation. There have been many ways to monitor bone loss, including radiographic indices, photon absorptiometry, and computed tomography. There is a reproducible association of fracture risk with dualenergy x-ray absorptiometry (DXA) measurement of bone mineral density (BMD), such that fracture risk doubled for each standard deviation (SD) decrease in BMD. This led the World Health Organization (WHO) to establish a scale for diagnosis of osteoporosis and osteopenia [15]. Osteopenia is defined as a value for BMD that lies between 1 and 2.5 SD below the young adult mean value. Osteoporosis is defined as a value for BMD that is more than 2.5 SD below the young adult mean value. Severe or established osteoporosis is defined as a value for BMD more than 2.5 SD below the young adult value in the presence of one or more fragility fractures. Reference young adult mean values are specific for men and women, for different countries, and for different racial and ethnic groups. These diagnostic criteria remain the guides for treatment decisions. An advance that takes other patient factors into account (age, family history, previous fracture, glucocorticoid use, smoking, alcohol use) are seen in the nation- and race-specific FRAX calculator:
[http://www.shef.ac.uk/FRAX/tool.jsp?locationValue=9], a tool that estimates the 10-year probability of fracture for an individual.
Age is a major risk factor for osteoporosis (Table 1). Agerelated changes in bone tissue contribute to its loss of mechanical properties [16]. The mechanical properties of samples of cortical bone decrease by 7-12% per decade in fracture toughness [17]. There are age-related changes in collagen that generate advanced glycation end products (AGE) associated with mechanical deterioration [18]. Further, bone and blood from osteoporotic fracture patients have a higher content of AGEs than do samples from controls. There are also age-related changes in the mineral component of bone that result in accumulation of larger, dense crystals that make bone more brittle with decreased fracture toughness [19]. Micro architectural changes with age contribute to bone quality and fracture risk; these include loss of trabecular connectivity and thickness, increases in cortical porosity, and accumulation of micro cracks.
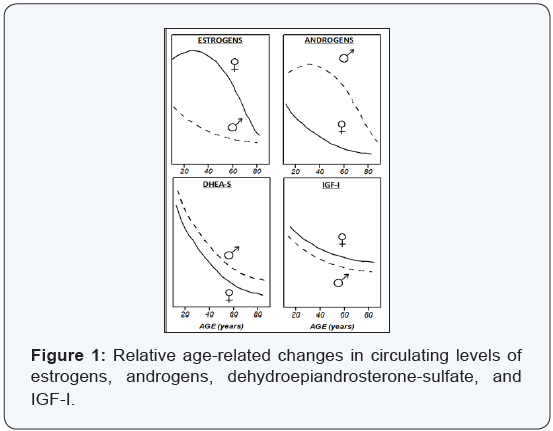
Bone-active hormones play multiple pathophysiological roles in osteoporosis. Women and men undergo multiple aspects of endocrinologic aging that have impacts on bone metabolism (Figure 1). To different degrees, both men and women experience menopause, andropause, and adrenopause, and their effects upon the skeleton [20]. The abrupt post-menopausal loss of bone mass is primarily due to increased resorption unfettered by estrogen, and the slower age-dependent phase results mainly from impaired bone formation due to loss of osteoanabolic activities. Estrogen suppression of osteoclastic bone resorption entails reduction of pro-resorptive factors including Interleukin-6. Age-associated declines in circulating estrogens, androgens, and adrenal dehydroepiandrosteronesulfate contribute to decreases in bone formation that are mediated locally through IGF-I.
Origin of the Inverse Hypothesis
Both OA and OP are recognized as age-related skeletal disorders, but it is commonly held that these diagnoses do not often occur together in the same patient. In 1972, Foss & Byers [21] noticed the absence of radiographic osteoarthritic changes in hips of osteoporotic patients with hip fractures [21]. That study was not well-matched for age or gender, but was taken as evidence for an inverse relationship between the two diseases, OA and OP. It remains widely held that “osteoarthritis protects against hip fracture” [22]. The hypothesis was appealing because of the observations that patients with a high body mass index (BMI) are at risk for OA and those with low BMI are at risk for OP.
The most recent systematic literature review of the relationships between hip fracture and OA at various joints was limited in drawing any conclusions because of the small numbers of eligible articles, different case definitions, only moderate agreement among reviewers, and heterogeneity in numerous covariates [23]. Those authors indicated the need for studies with evaluation of joints by magnetic resonance imaging (MRI) and evaluation of bone by BMD.
To be sure, BMD measured at the femoral neck of OA hips was significantly greater than for age-matched non-OA subjects [24], but this does not address the question of systemic OP. A detailed twin study found increased BMD only at the OA-affected site and not at other sites [25].
Coexistence of Osteoarthritis and Osteoporosis
Surgeons use radiographic indices of bone quality for arthroplasty treatment planning, knowing that poor bone quality is a determinant of intraoperative fracture risk and poor prosthesis longevity. The Dorr classification of femoral geometry distinguishes three types of bone (A, B, C) and a measurement of cortical thickness to characterize the quality of the implantation site [26]. There are strong correlations between bone types and cortical thickness that were validated by histomorphometric indices of bone microarchitecture. It is notable that 63% of the osteoarthritic men and women studied had radiologic and histomorphometric evidence of osteopenia. More recently, we found strong correlations between BMD T-scores and Dorr radiographic parameters in a cohort of OA women [27].
BMD studies of OA subjects provide consistent evidence of the coexistence of OA and OP [2]. We initiated a study to characterize women presenting for hip arthroplasty for OP hip fracture, compared with OA subjects scheduled for hip arthroplasty as a non-fracture control group [28]. An unexpected finding was that 25% of OA subjects met the WHO criterion for OP (having BMD T-score of less than -2.5). They also had elevated markers of bone turnover, statistically equivalent to those with OP fracture [29]. These findings clearly reject the hypothesis that all OA women are protected against bone loss and OP risk. Vitamin D-deficiency was common in both groups of OA women with and without occult OP. OP was found even in the early post-menopausal period, but there was a significant effect of years-since-menopause on T-scores and bone turnover markers. In addition, OA subjects with lower weight or lower BMI had poorer quality of bone by both radiographic and DXA measures.
Subsequent BMD studies of subjects with advanced OA revealed OP in 21-32% of women and men. A Finnish study reported 28% of OA women had OP and 45% had osteopenia (BMD T-score between -1 and -2.5) [30]. A German study revealed OP in 29% of women and 20% of men with OA, with age as a significant risk factor [31]. In that study, 37% and 43% of the male and female patients, respectively, had osteopenia. A British BMD study reported that 23% of patients with endstage OA had evidence of OP at one or more sites and a further 43% of patients had osteopenia at one or more of the sites measured [32]. A Brazilian study reported that 21% of OA men and women awaiting hip arthroplasty had osteoporosis and 38% had osteopenia [33]. A Slovakian study detected 32% of hip arthropathy subjects with osteoporosis and 21% with osteopenia; lower BMI was found to be associated with osteoporosis [34]. A number of generalizations can be drawn from our and others’ studies with OA subjects awaiting arthroplasty. Most groups reported a high incidence of vitamin D-deficiency in their cohorts, but not significantly greater in the OP groups. Advanced age and low BMI are major discriminating factors for OP in OA subjects.
There is limited information about rates of bone loss in OA and non-OA subjects. A large longitudinal cohort of communitydwelling subjects between the ages of 50 and 80 years was used for analysis of changes in hip BMD over 2.6 years and correlation of those changes with baseline hip and knee OA [35]. Non-OA and OA subjects had equivalent baseline hip BMD scores. Subjects with radiographic hip or knee OA had significantly greater total hip bone loss, as much as 3.6-fold. The authors considered it unlikely that differences in mobility accounted for the findings because there were equivalent changes in pain scores and in numbers of steps taken per day (by pedometer).
A different approach by investigators in Norway entailed analysis of OA in patients with a hip fracture, compared with patients hospitalized for hip contusion, the fracture being considered by the authors as an indication of greater bone fragility [36]. Finding similar prevalences of radiographic OA in those two groups led the authors to reject the hypothesis that OA protects against sustaining a hip fracture.
Some information is available from subjects with shoulder OA [37]. Based upon BMD T-scores at the femoral neck, 12% of 230 women and men were osteoporotic and 46% were osteopenic. Several predefined risk factors were associated with low hip BMD in subjects with shoulder OA: female sex, advanced age, sedentary lifestyle, prior fracture, family history of fractures, thyroid replacement therapy, and inflammatory joint disease. Hounsfield unit (HU) measurements from shoulder CT scans were found to be significantly correlated with femoral neck BMD. The investigators urged surgeons to use humerus HU and patient characteristics to identify and refer patients at risk for osteoporosis.
A large longitudinal cohort of Australian men and women was used to examine the effect of BMD on the association with self-reported OA and with fracture incidence during the follow-up period of 0.1 to 22 years [38]. Consistent with many previous findings, prevalence of OA increased with each higher BMI and femoral neck BMD category. Fractures were identified by radiological evaluations and were found to be significantly greater for women, but not men, who had self-reported OA. Both OA women and men had greater reported falls than did the non-OA women and men, and falls may have some part in the observed OA-fracture relationship for women. The associations were stronger for vertebral and limb fracture than for hip fracture. Additional inexplicable findings were that fracture incidence was not elevated in women with osteoporotic BMD scores and that OA-hip fracture relationship disappeared upon adjustment for age, BMI, and hip BMD. It appears that the OA women were older than the non-OA group and that OA was not in advanced stages. More details are needed about OA diagnosis, joints involved, duration of OA, and nature of the falls in order to interpret those findings. A smaller survey of Canadian women and men scheduled for hip or knee arthroplasty indicated OP in 26%, as defined by reporting a physician’s diagnosis, history of a fracture occurring as a result of a fall from standing height or less, or current treatment for OP with bisphosphonates [39].
Clinical Implications of Osteoporosis in Osteoarthritic Patients
It is critical for surgeons to recognize the quality of bone in patients undergoing surgery for advanced OA. There are great implications brought about by OP in OA both in consideration of joint replacement and complications following joint replacements. A major consideration of initial fixation of uncemented implants in OP bone is that any micromotion would decrease construct stability and thus interfer with ingrowth at the implant/bone interface. There is risk of periprosthetic fracture due to the unfavorable combination of osteoporosis, stress shielding, and the differences between the modulus of the implant and the bone. The Dorr classifications are useful for surgical planning in cases of Dorr type C (osteoporotic) bone where use of cement may be indicated. Methylmethacrylate cement has a modulus between cortical and cancellous bone and can mollify the differences between sclerotic and OP or osteopenic bone.
OP can produce specific complications in an obese female with varus osteoarthritis of the knee. The patient may have general osteoporosis and sclerosis on the medial side. If there is significant core obesity and a long moment of increased load, the patient may be better served if the tibial component has a longer stem to prevent collapse of the tibial surface.
Studies that included bilateral hip BMD for subjects with unilateral hip OA raise warnings about BMD screening for OP in the general population [28,30,40,41]. In all studies, femoral neck BMD or BMC measurements were significantly higher in the OA hip than contralateral, non-OA hips for both women and men. Because measurements of BMD are commonly done in only one hip (usually the non-dominant), bilateral differences in subjects with occult hip OA may result in differences in osteoporosis classification and treatment decisions. In fact, the calculated probabilities for total or hip fracture according to the FRAX calculator were confounded by the presence of OA [42]. Thus, clinicians who perform bone density testing should assess the possibility of OA in 1 hip and measure the BMD on the contralateral hip.
Conclusion
A subset of OA patients with advanced age and low BMI may have low bone density at non-OA sites and if left untreated may be at risk of fragility fracture.
To Know More
About Orthopedics and
Rheumatology Open Access Journal Please click on:
For more Open Access
Journals in Juniper
Publishers please click on:
https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on:
https://juniperpublishers0.wixsite.com/juniperpublishers

Comments
Post a Comment