Juniper Publishers - Clinical Review about the Role of Platelet Rich Plasma for the Treatment of Traumatic and Degenerative Musculoskeletal Disorders
Orthopedics and Rheumatology Open Access Journal
Abstract
The use of orthobiologics compounds is rapidly expanding in the field of orthopedics and sports medicine. Platelet rich plasma (PRP) represents the second generation of ortobiologics that has numerous advantages as an autologous blood derivate for the treatment of traumatic and degenerative musculoskeletal diseases. Platelet is naturally involved in haemostasis and tissue healing processes due to their content in growth factor and other bioactive molecules. Basic science and preclinical evidence supports the use of platelet derived growth factors as well as of PRP for enhancing reparatory processes in musculoskeletal tissues. Clinical results about the use of PRP for bone, tendon, cartilage or muscle healing are encouraging and continue to accumulate in the recent years. Proteomic profiling and biomarker based PRP characterization have the potential of advancing the field of PRP application. High quality studies are awaited in order to enable clear cut therapeutic indications
Keywords: Platelet rich plasma; Orthopedics; Tendon; Osteoarthritis; Bone; Muscle
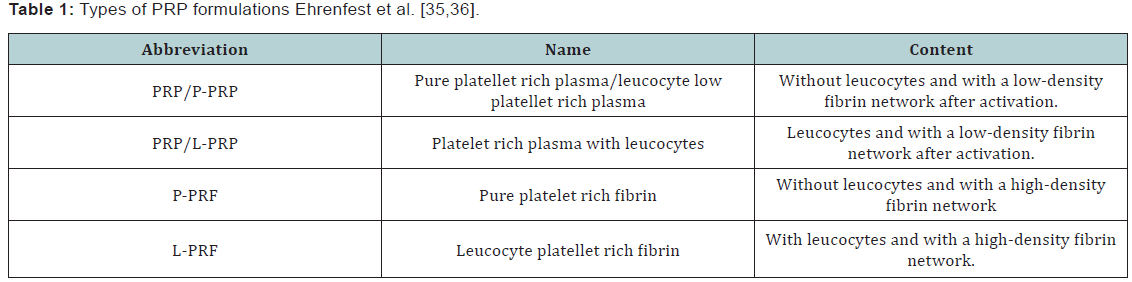
Abbreviations: PRP: Platelet Rich Plasma; BMP: Bone Morphogenetic Protein; HA: Hyaluronic Acid; WBC: White Blood Cells; RBCs: Red Blood Cells; ADP: Adenosine Diphosphate; ATP: Adenosine Triphosphate; TGF: Transphorming Growth Factors; PDGF: Platelet Derived Growth Factor; ECM: Cell-Extracellular Matrix; FGF: Fibroblast Growth Factors; IL-1: Interleukin -1; MSCs: Mesenchymal Stem Cells; TNF-α: Tumor Necrosis Factor α; NFκβ: Nuclear Factor Kappa-Beta; 3D: Three Dimensional; ADSC: Adipose Derived Stem Cells; ACT: Autologus Chondrocyte Implantation Techniques; GF: Growth Factors; PRF: Platelet Rich Fibrin Products; OA: OsteoArthritis; KL: Kellgren-Lawrence; IA: IntraArticular; IKDC: International Knee Documentation Committee; KOOS: Knee Injury and Osteoarthritis Outcome Score; MRI: Magnetic Resonance Imaging; HHS: Harris Hip Score; VAS: Visual Pain Analogue Score; OCL: OsteoChondral Lesions; PCL-TCP: PolyCaproLactone-20% TriCalcium Phosphate; TLIF: Trans-foraminal Lumbar Inter-foraminal Fusion; VEGF: Vascular Endothelial Growth Factor; HGF: Hepatocyte Growth Factor; RCT: Rotator Cuff Tear; ACL: Anterior Cruciate Ligament Reconstruction; DASH: Disabilities of the Arm; Shoulder and Hand; CRAT: Chronic Recalcitrant Achilles Tendinopathies; MRSA: Methicillin-Resistive Staphylococcus Aureus
Introduction
The use of orthobiologics is expanding at a rapid pace in the field of bone and joint surgery, tendon and wound healing [1]. While a precise definition has not been elaborated, orthobiologics are considered to be the naturally occurring elements that are used in order to initiate, augment or modulate healing of bone, joints, tendons, ligaments, muscles and/or cutaneous defects. Among the biological compounds currently considered as orthobiologics are included the bone grafts of various origins, autologous blood and conditioned serum, platelet rich plasma (PRP), growth factors and stem cells. Some of these factors such as bone grafts or autologous blood have a long history of use in orthopaedic and/or rheumatologic settings. The use of platelet rich plasma (PRP) or stem cells has been initiated with the beginning of the third millennium and is currently in different stages of penetrating clinical practice. Taking advantage of cutting edge research and using advanced technologies, orthobiologics are processed or engineered to respond to a certain clinical need.
The era of orthobiologics is considered to originate in the pioneering discovery of bone morphogenetic protein (BMP), the first growth factor to be described. Marshal Urist [2] an orthopedic surgeon, isolated BMP from demineralized bone matrix demonstrating its role in bone healing of fractures and nonunion. The modern use of orthobiologics has been stratified by some authors in three stages of increasing complexity as referring to the intrinsic mechanism of action. The first generation is represented by viscosupplementation with hyaluronic acid (HA), the second stage involves the use of PRP while the third and most advanced stage consists in cell based therapies and the use of growth factors [3,4]. In the following we will introduce basic science motivating the use of PRP further presenting the current status of the use of PRP in orthopaedic practice in the field of cartilage, tendon and bone healing.
Platelet rich plasma for musculoskeletal healing
PRP is a plasma suspension derived from whole blood containing variable amounts of platelets [5] Depending on the preparation process PRP might contain as well white blood cells (WBC) and red blood cells (RBCs). Platelet content ranges from 2 to 6 fold above baseline, making PRP a valuable source of concentrated autologous platelets. PRP is usually prepared from autologous blood using extracorporeal blood processing methods such as cell savers/separators, centrifugation or filtration [6]. The large variability of blood processing methods result in plasma samples with variable composition and platelet content that inevitable influences the biological effect [7].
PRP was used for the first time in 1987 as a blood substitute during open heart surgery [8]. In 1990 an autologous fibrin sealant (fibrin glue) obtained by polymerization of fibrinogen with thrombin or calcium chloride [9] was introduced as a topical hemostatic while the first preparation of an autologous PRP product from a small quantity of blood was described in 1999 [10]. Initially used in dental and oral and maxillofacial surgery, PRP use has spread in various fields from sports medicine to cosmetics, orthopedic surgery and ophthalmology. The relatively low cost, easiness in use as well as massive commercial involvement has facilitated PRP rapid expansion in medical practice. As with every relatively new method, the use of PRP has opponents and advocates. There is a strong basic science motivation for the use of platelet concentrate as a healing promoter and/or enhancer, however, evidence from welldesigned clinical trials to support specific clinical indications are only beginning to accumulate.
Basic science- platelets and their role in hemostasis and tissue healing
Platelets are the smallest cellular components of blood. With a diameter ranging from 2-6 μm, platelets are a-nucleated but do have, however, cellular organelles such as mitochondria, a contractile cytoskeleton and intracellular vesicles. Platelets are formed in the bone marrow representing fragmented parts of cytoplasm from megakaryocytes differentiated from a myeloid precursor. Platelets contain among intracellular vesicles dense and alpha granules. Dense granules content consists in calcium, serotonin as well as Adenosine diphosphate (ADP) and Adenosine triphosphate (ATP) molecules. Alpha (α) granules are formed during megakaryocyte stage; contain clotting factors as well as more than 30 types of growth factors, cytokines and other proteins [11]. Platelet membrane is folded and contains an interconnected network of canaliculi. In normal resting state, platelets have a round shape and are not thrombogenic. Upon activation platelets spread their membrane forming pseudopodia, aggregate and release their granular content through canaliculi system exerting their role in haemostasis and wound healing.
Haemostasis involves the balanced action of local vasculature, plasma factors as well as platelets. After an injury, blood vessel walls contracts, the exposed sub endothelial collagen binds the plasmatic Von Willebrand factor facilitating platelet adhesion and activation. Other two mechanisms are the Thromboxane A2 from arachidonic acid within the phospholipidic layer of cellular membrane and thrombin activation. Upon activation platelets release their granular content resulting in the formation of initial clot plug. The second haemostasis stage involves the formation of fibrin from blood fibrinogen by activation of the coagulation factors cascade. Fibrin network stabilizes the platelet plug consolidating the clot. The third haemostasis step involves the activation of WBCs that release fibrinolytic cytokines that will produce clot lysis and blood vessel re-permeabilization after healing [12].
Wound healing is a complex event that involves intercellular, cell-extracellular matrix (ECM) interaction as well as growth factors and cytokines. The type of healing response and efficiency depends on the extent of injury and wound type. In this process, platelets and platelet released growth factors such as platelet derived growth factor (PDGF) have a significant role. Basically wound healing begins with blood clotting process and local haemostasis. Further on, in the following 2-3 days, inflammation is produced by migration of blood neutrofils and subsequently of tissue resident macrophages. Activated macrophages release growth factors such as members of transphorming growth factors (TGF) family, fibroblast growth factors (FGF), PDGF, interleukin -1(IL-1). After third day, local angiogenetic processes as well as fibroblast proliferation begins, followed by ECM collagen deposition after day 5. Wound epithelization in the case of skin injuries and tissue remodelling concludes the healing process that can last 10-14 days depedingly on anatomic location and host dependent parameters [13]. Platelet derived growth factors are therefore involved in multiple stages of wound healing starting with degranulation process and inflamation, to matrix deposition, colagen production and reepitelization. It is important to note that an important part of the growth factors contained by the α granules have receptors on various musculoskeletal tissues justifying their use for enhancing healing of these structures.
In the process of fracture repair and calus formation (bone healing) platelet derived growth factors exert a stimulatory action on bone cells. Bone growth, turnover and repair after fracture or in surgically induced fusion processes represents an interplay between the activity of cellular elements and numerous biochemical and biomechanical factors. Cells (osteoblasts, osteoclasts, osteocytes, osteoprogenitor cells, and the hematopoietic component in the bone marrow) cooperate in matrix deposition, resorbtion and remodeling [14]. Similar with the wound healing process, fracture repair and calus formation incorporates an innitial stage of clot formation, followed by inflamation, proliferation and remodelling. At fracture sites, platelet degranulation release PDGF, members of TGF-β family, EGF, that are present as well in bone and cartilage. Chondrocytes and osteocytes are enriched in TGFβ1 receptors [15] while a combination of PGF, TGF- , FGF, and EGF has been found to stimulate osteoblast differentiation to mature osteocytes [16]. Platelet derived growth factors are involved in bone healing in by three mechanisms: during osteogenesis induce the presence and proliferation of osteoprogenitor cells within the fracture area, participate to osteoinductive process by stimulating progenitor differentiation to mature osteocytes being involved as well in osteoconduction. Osteoconduction requires the presence of a natural or synthetic scaffold acting as a ECM (a natural autologus or allogeneic bone graft of a syntethic cone substitute). Platelet derived growth factors, especially PDGF was shown to be involved in chemotaxis of stem cells, mitogenesis and differentiation, contributing to graft population and de novo bone formation [17]. This supports the use of PRP for enhancing bone repair in fractures, in combination with bone grafts in non or delayed unions and bone fusion procedures.
Cartilage repair and regeneration Cartilage lesions, traumatic or degenerative, are challenging to treat due to the inherent tissue structure with a poor cellularity and lack of vascularity that does not allow for innitiation of classical wound healing processes [18]. In vitro and in vivo studies on the effect of different PRP formulation or platelet derived growth factors are available (for a systematic review of basic science of cartilage repair using PRP [19]. PRP was found to increase chondrocyte and mesenchymal stem cell (MSCs) proliferation [20] and to increase cartilage ECM compound synthesys (proteoglycan, glycosaminoglycan, and type II collagen deposition) [21]. In inflmatory conditions, in the presence of IL-1β, tumor necrosis factor α (TNF-α) or nuclear factor kappa-beta (NFκβ), PRP partially decreased the inhibitory effect of inflamation on collagen II and aggregan gene expression [22] with strong restoration of type II collagen and proteoglycan from the inhibition of IL-1β+TNF-α in a three dimensional (3D) model in the presence of collagen matrix [23].
Evidence from animal studies using PRP formulation as adjunct therapy in focal cartilage repair procedures reported histological improvment of repair tissue [24] while others reported worsening gross apearance and histological scores compared to untreated group [25]. ECM matrix deposition proteoglycan [26] or collagen II content of repair tissue [27] increased in the PRP treated groups compared to control. PRP was found to increase gross and histologic appearance of focal defects treated with PRP conditioned adipose derived stem cells (ADSC) pointing toward a method for enhancing chondrogenesis [28]. In vivo studies using PRP for treating osteoarthritis or inflamatory arthritis reported the increase of proteoglycan mRNA levels, cartilage macroscopic and histologic appearance as well as attenutation of synovial and cartilage inflamation. The pro inflamatory environment of arthritic joints could be modulated by platelet growth factor release and PRP administration [29]. It has been proposed that PRP application could improve cartilage repair after bone marrow stimulation techniques by improving subchondral plate derived MSCs chondrogenesis [30]. PRP could be used as well in combination with scaffolds when repairing chondral or osteochondral defects or in combination with autologus chondrocyte implantation (ACT) techniques [31].
Clinical results regarding PRP application
The main rationale for using PRP in clinical practice is to deliver a concentrate of platelet derived proteins including growth factors (GF) that assist and enhance the reparative processes. The ease of preparation of an autologus blood derivative at the time of surgery or application is appealing. In an appropriate laboratory, operating theatre or even in an appropriate room of an outpatient clinic facility, PRP can be prepared in the extent of couple of minutes using commercially available equipments from blood collected by venous puncture using an anticoagulant. Non coagulated blood is used mostly for preparation of fibrin and/or platelet rich fibrin products (PRF) [32]. PRP can be delivered via open or arthroscopic surgery during various orthopedic procedures as a step of a ligament, meniscal, tendon or muscle repair. PRP can be mixed with bone or ligament grafts, and is usually activated in order to form a gelatinous mass that is easier to handle during open surgery. Minimally intervention procedures in the form of injection therapy using fluid PRP can be performed by a sports medicine, rheumatologist, physical therapist or orthopedist. Injectional therapy is preferably performed under ultrasound guidance to maximize results [6,33]. PRP prepared from blood collected on anticoagulant can be activated at the preparation time. For activation, calcium chloride, autologus prepared thrombin or soluble collagen type I are preffered to bovine thrombin products due to risck of inducing coagulopathy [34]. Collagen activation might be prefferable for preserving growth factor avaialbility than thrombin [35]. Other oppinions advocate the use of inactivated PRP since platelets can be activated by the contact with the tissue to be treated. From platelet granules 95% of growth factors are relased during the first hour post preparation. In the following 5-7 days platellets secrete and release additional growth factors. Different types of PRP preparation exist and a working classification based on platellet and fibrin content is currently accepted and validated [36,37] (Table 1).
Clinical application of PRP in joint healing
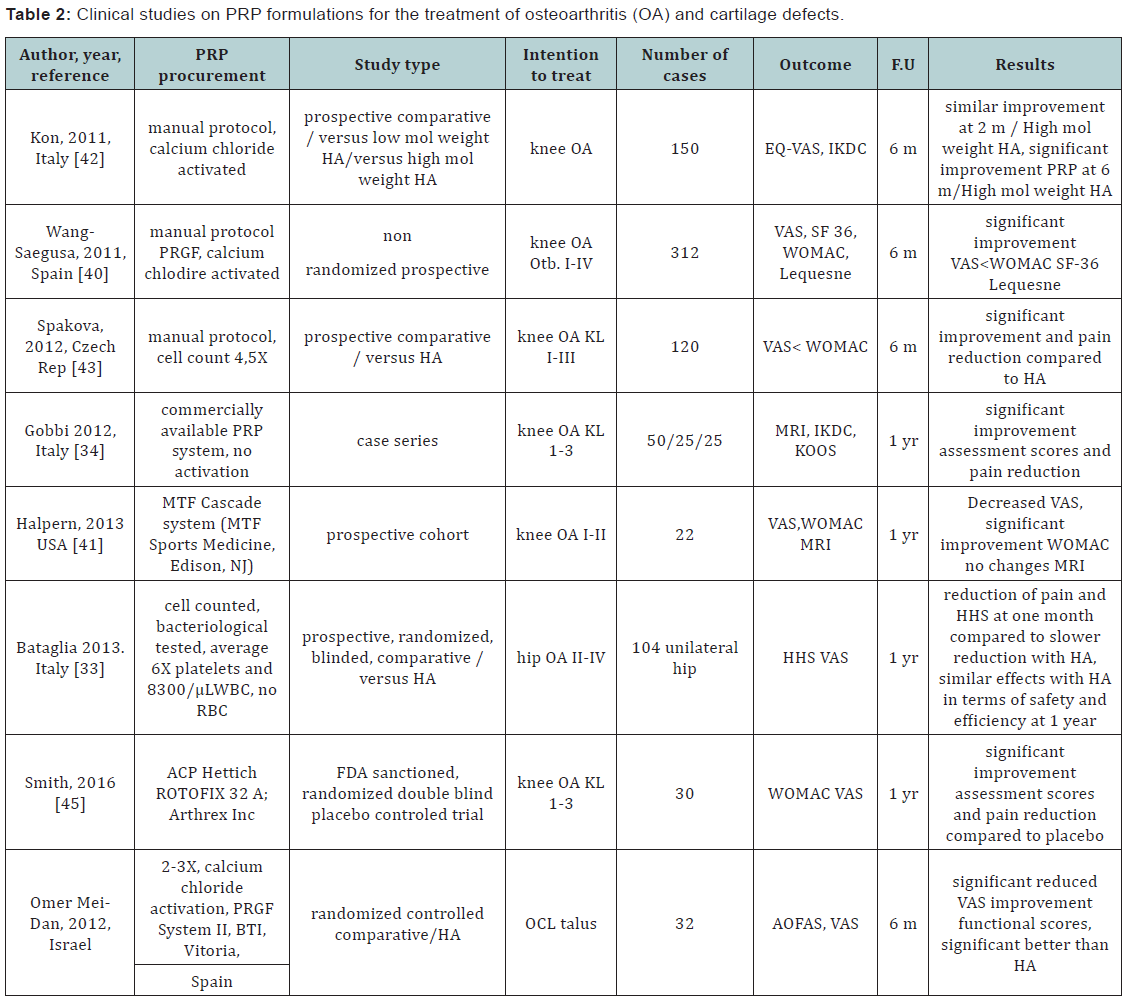
Joint environment requires a delicate balance of catabolic and anabolic factors that promote development, turnover and repair. The currently definition and treatment orientation focused mainly on cartilage pathology is giving way to a more integrative approach conssidering joint as a complex organ componed of subchondral bone, synovial tissue, fatty sinovium, subcutaneous fat as well as cartilage, intraarticular tendon and menisci and periarticular ligaments [38]. The intraarticular use of PRP products could act simoultaneously in a concerted manner to restore protein synthesis to rebalance metabolic pathways that are disturbed in post traumatic, degenerative or inflamatory joints. It has been used to treat cartilage lesions, to prevent posttraumatic arthritis and to retard progression in osteoarthritis and rheumatoid or psoriasic arthritis. In a systematicc review including 59 papers of which 22 clinical studies, Filardo et al. [39] concluded that the existent clinical evidence denotes overall good outcomes and no adverse effects. Instalation of results as well as the reported clinical benefits are more likelly be the result less of cartilage restauration but more of overall joint metabolic balancing. Thus, tissue regeneration in itself might not be the predominat mechanism of PRP action thac could rather induce reduction of inflamatory mechanisms. Reduced inflamatory cell chemotaxis toward symovium and periarticular tissue has as result decreased pain and increased mobility [39]. In a case series of 50 active patients with knee osteoarthritis (OA) grade 1-3 Kellgren-Lawrence (KL) were treated with 2 intraarticular (IA) injections at 1 month interval of autologous PRP were followed up to 1 year using International Knee Documentation Committee (IKDC) subjective and objective score, Knee injury and Osteoarthritis Outcome Score (KOOS) and magnetic resonance imaging (MRI).
All patients significantly improved in terms of pain and reported quality of life [34]. In a prospective, randomized, comparative clinical trial enrolling 104 patients with unilateral hip OA followed up over 12 months, autologous PRP was delivered in three doses over two weeks interval under ultrasound guidance. Harris hip score (HHS) and visual pain analogue score (VAS). Compared to the use of hyaluronic acid (HA), PRP was proved to be as safe and efficacious as HA at 12-month follow-up in terms of functional improvement and pain reduction [33]. A non-randomized, prospective study on 312 patients with knee OA and Outerbridge I-IV chondropathy were treated with three IA PRP doses at 2 weeks interval. Significant improvement in pain and functional parameters were recorded at 6 months post last injection [40,41]. A prospective study compared the use of PRP versus high and low molecular weight HA in 150 patients with knee OA. At 2 months interval, similar improvements in terms of pain and function was recorded in PRP and high molecular weight HA groups, however PRP group showed significant improvement at same parameters at 6 months follow up [42]. It has been argued that to date the power as well as quality of the studies being limited the role of PRP injections in the treatment of OA is still unclear [43,44]. However, high level evidence studies are beginning to accumulate supporting the use of PRP formulations for OA treatment. In a FDA sanctioned, double blind, placebo controlled randomized study, PRP administration improved WOMAC scores by78% from the baseline score versus only 7% for the placebo control group after 1 year with no adverse effect. Study concluded PRP is safe and benefits patients with knee OA [45].
The presence or absence of leucocyte fraction within the PRP preparation is a factor that influences results. A metaa-analysis including 6 randomized controlled trials and 3 prospective comparative studies compared clinical outcomes and rates of adverse reactions between LP-PRP and LR-PRP for the treatment of knee OA. The study concluded that there is sufficient evidence to state LP-PRP improves functional outcome scores compared with HA and placebo, both LR-PRP and LP-PRP being safe [46]. PRP has been used as well for the treatment of cartilage defects. A randomized controlled trial evaluated the safety and efficacy of IA injections of PRP compared to HA for the treatment of osteochondral lesions of the talus (OCL). Pain reduction and functional improvement at short time follow up (6 months) was significant higher for the PRP group, recommending the procedure for the treatment of OCL with this location [47].
When used as an adjunct therapy, in combination with microfractures for the treatment of OCL, PRP resulted in resulted in improved functional score status in the follow up time (medium 16, 5 months) The study concluded that further investigations will be required to determine the long-term efficacy of this approach [48]. In a randomized prospective controlled study the effect of PRP versus HA as adjunct therapy for microfracture in OCL was investigated with a medium 15,3 months follow up. Both PRP and HA injections improved the clinical outcomes and can be used as adjunct therapies to treating OCL with microfracture. Because a single dose of PRP provided better results, PRP was recommended as the primary adjunct treatment option in the talar OCL in the postoperative period [49] (Table 2).
PRP in bone regeneration
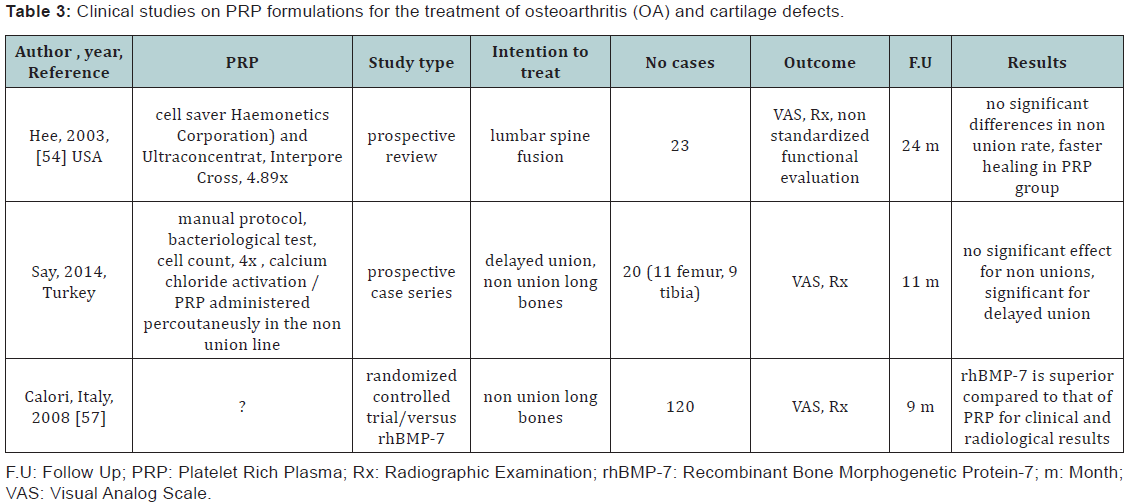
PRP is used predominantly in maxillofacial surgery as an additive to autologus or synthetic bone grafting. For the orthopaedic practice, its use remains limited mainly due to the current lack of well documented evidence based medicine as well as of clinical treatment algorithms. PRP has been used as a co-adjuvant method for enhancing union of long bones (acute fractures, pseudoarthrosis) and in bone defect grafting. Results from animal studies are controversial. One study investigating the healing 8 mm femoral non unions in rats using polycaprolactone-20% tricalcium phosphate (PCL-TCP) composite scaffolds, mixed with PRP reported accelerated early vascular ingrowth and improved longer-term functional graft integration compared to PCL-PCT only [50]. Other studies are reporting no beneficial efects when using PRP combined with collagen sponge for the healing of calvarial defects in rats [51] or limited regenerative potential when mixed with xenogeic bone grafts for treating mandibular defects in dogs [52].
Two randomized prospective clinical trials with a total of 148 cases, published before December 2011 were evaluated. One of the studies compared recombinant human BMP-7 (rh- BMP-7) versus PRP for the treatment of pseudoarthrosis, the other compared the union of valgising tibial osteotomies in three conditions (PRP, PRP plus mesenchymal stem cells and no adjuvant therapy). The evaluation concluded that the studies had low power and moderate to high risk of bias not being able to support the use of PRP as an adjuvant therapy for these indications [53].
A prospective review of 23 patients who underwent transforaminal lumbar inter-foraminal fusion (TLIF) with PRP with a minimum 2-year follow-up concluded non-significant differences between PRP treated group compared to historical non-treated lot, however, faster healing and bony fusion could be reported in the PRP group [54]. A study using PRP as adjuvant modality to prevent syndesmosis non-union during total ankle reconstruction using DePuy Agility system, reported statistically significant improvement in the 8- and 12-week fusion rates as well as significant reduction in delayed unions and non-union in the PRP group [55]. PRP has been investigated as a method for percutaneous treatment of enhancing long bone healing for clinical applications. It is proposed to be an efficient method to address delayed union, however only limited results can be obtained for nonunion and only in selected cases [56]. The essential factor is reported to be the average time from the initial surgery to PRP injection for non-union, less than 11 months seems to be critical for good outcomes. A prospective study investigating the role of fluoroscopic guided percutaneous injection of PRP for selected cases of delayed unions or nonunions of long bones (femur or tibia) concluded that sufficient union could not be induced by PRP administration in the case of non unions. However, in selected patients with delayed unions of long bones PRP can be reccomended to augment the preexistent fracture fixation methods (intramedular nail or plate fixation) [57,58].
In a prospective randomized study the efficacy of PRP was compared to the use of rhBMP-7 in combination with autologus bone graft in 120 patients with tibial, femoral, humeral radial and ulnar non unions with a maximum 9 months follow up. The study concluded that the application of rhBMP-7 as a bone-stimulating agent is superior compared to that of PRP with regard to their clinical and radiological efficacy. Evidence accumulated in the recent years point toward a necessary effort to standardize PRP procurement protocols, therapeutic formulations, dosage, and timing of application as well as modalities of reporting clinical outcomes. This will derive in accumulation of high quality clinical evidence required for establishing if there is a role for PRP use as bone healing stimulator in orthopedic applications (Table 3).
PRP for tendon healing
PRP treatment for tendon and ligament injuries and degeneration was one of its earliest use for musculoskeletal applications. In vitro studies support the mitogenic activity of PRP on tenocytes, the stimulatory effect on their ECM protein production. Moreover, PRP promotes expression of angiogenetic factors such as vascular endothelial growth factor (VEGF) or hepatocyte growth factor (HGF) by tenocytes contributing to healing process [6]. Growth factors in PRP cocktail were proven to exert anabolic effects, increased chemotaxis of bone marrow cells, improved histologic organization, and increased force at failure in vitro as well as in animal models [59,60]. The anticatabolic effect of TGF-β known to inhibit expression of potent catabolic factors such as IL-1β and TNF-α as well as of matrix degradative enzymes might have a role in protecting tendons from degradative processes.
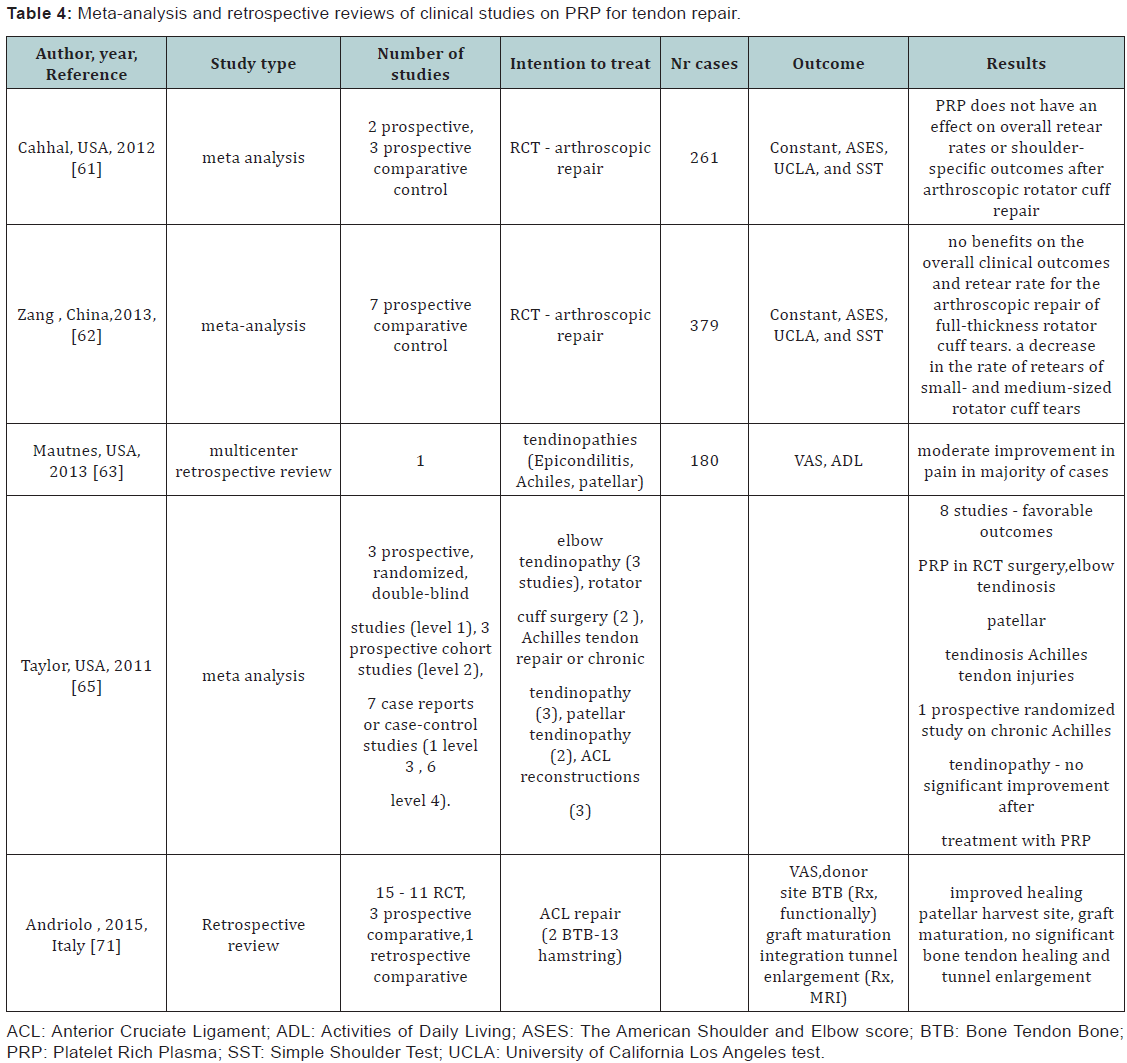
Several clinical studies report about the use of different PRP formulations as injection therapy or as tendon repair augmentation procedure. Revising the results from 2 randomized and 3 non-randomized with comparative control studies investigating the role of PRP as augmentation procedure for complete rotator cuff tear (RCT), Cahhal et al. [61] concluded that PRP does not have an effect on overall re-tear rates or shoulder-specific outcomes after arthroscopic rotator cuff repair [61]. A meta-analysis including sevens studies on 379 patients undergoing arthroscopic RCT procedures with and without PRP application found no benefits on the overall clinical outcomes and re-tear rate. There was, however, a decrease rate of re-tears among patients treated with PRP for small- and medium-sized rotator cuff tears but not for large- and massive-sized tears [62].
In a multicenter retrospective review on 180 cases investigated the role of ultrasound guided injections in treating tendinopathies (most common sites lateral epicondyle, Achilles, and patellar tendons). Majority of the patients reported moderate pain improvement, 95% of patients having no pain at rest 68% reported no pain during activities, and 85% of patients were satisfied with the procedure [63]. Another study investigated the effect of PRP application at the site of patellar tendon harvest for anterior cruciate ligament reconstruction (ACL). PRP was reported to increase healing of patellar tendon harvest site as assessed by MRI after 6 months and reduced pain in the immediate postoperative period. However, isokinetic testing results were not different between the PRP treated and non-treated groups at 6 months [64]. In a systematic review, the role of PRP in treating tendon injuries and tendinopathies was investigated. PRP was used for patellar (2 studies) and elbow tendinosis, (3 studies) Achilles tendon injuries (3 studies) rotator cuff repair (2 studies) and for augmenting ACL reconstruction procedures (3 studies).
The type of the studies investigated were 3 prospective, randomized, double-blind, 3 were prospective cohort studies and 7 were case reports or case-control studies. Eight of the studies investigated reported favorable outcomes after the use of PRP as augmentation in rotator cuff surgery, injection in elbow tendinosis, patella tendinosis, and Achilles tendon injuries (repair after acute tear and revision surgery), one prospective randomized controlled study showed no significant improvment in PRP application as injection therapy in Achiles tendonopathy.
A large variability in the modality of obtaining PRP, the volume of blood collected, the activation methods as well as modalities of application (injection, gel, fibrin membrane scaffold) making the results difficult to compare. The meta– analysis concluded that PRP application has advantages such as faster recovery, possible reduction of recurence and no adverse effects, however, more randomized controlled comparative studies are needed in order to ascertain the clinical efficiency in tendon healing. The optimal dosage, number and interval in the case of injection therapy needs to be further clarified. Special investigation are requiered in order to compare the use of liquid PRP to gel or scaffold/matrix basedd formulation relative to their potential additive effect [65].
Whenever the use of PRP for chronic overuse tendinopaties is more efficient than other existent treatment methods and whenever a certain anatomic location is more prone to be responsive, is still a question of investigation. To date, results from clinical studies report a moderate to medium effects in the treatment of elbow or Achiles tendinopathies. A multicentric randomized controlled trial compared the use of PRP and needling under local anesthesia compared to needling only for lateral epicondilitis in 230 patients (in 12 centers over 5 years). Even thought no significant differences could be detected at 12 weeks , at 24 weeks, clinically meaningful improvements regarding pain were reported for the PRP group [66]. A randomized controlled trial compared the use of PRP versus corticosteroids in 100 patients with elbow epicondilitis. Pain and functionality as assesed by Disabilities of the Arm, Shoulder and Hand (DASH) was found to be significantly improved in the PRP group exceeding the cortocosteroid effect even at 2 years interval. The authors concluded that in order to establish a clinical therapeutic algorythm, further investigation and follow up of the study are needed [67]. In a randomized controled trial comparing the efect of PRP to whole blood injection in 76 patients with lateral epicondilitis for maximum 12 months follow up concluded that no significant evidence could be detected between groups regarding pain and functionality [68].
In a retrospective study, intra-tendon administration of a single PRP injection was found to have significant role in improving pain and function in mid-portion Chronic Recalcitrant Achilles Tendinopathies (CRAT) over a median 50 months follow up with no adverse effects and significant lower tear rate [69]. In another retrospective study on 26 patients with Achilles tendinopathy that have undergone surgery with PRP administration or injection PRP treatment alone, showed significant degrees of improvement in pre-MRI and post-MRI imaging studies with no significant differences between the groups [70].
A systematic review included all clinical evidences on the use of PRP as a method for biological augmentation of ACL repair, Andriolo et al. [71] included 15 clinical trials, 1 randomized controlled, 3 prospective comparative studies, and 1 retrospective comparative trial. In the studies investigated PRP was used either to improve healing of patellar tendon (in bone patellar bone BPB procedures), to coat the intraarticular portion of the graft or administered within the bonny tunnels in hamstring procedures to enhance bone tendon healing. No adverse effects and even reduced surgical morbidity in two of the studies, better healing response of patellar tendon with BTB procedures as assessed radiologically or functionally. PRP might enhance graft maturation with no significant evidence on osteoligamentous healing or prevention of tunnel enlargement [71] (Table 4).
A preclinical study reports on PRP delivered in gelatin hydrogen efficient in improving avascular zone meniscal tears healling in rabbits [72]. Currently no study has been published on clinical results using PRP for meniscal repair while one registered clinical trial has been wihtdrawn prior to enrollment [73].
Antimicrobial activity of PRP
PRP posses antimicrobial activity due to WBC content, to intrinsic microbiostatic and microbiocidal effect of platelet α granules, as well as of complement or other heat-sensitive components within plasmatic fraction [5]. An in vitro study tested the antimicrobial activity of pooled PRP samples finding antimicrobial activity against Methicillin-resistive Staphylococcus aureus (MRSA) and E Coli [74]. Preclinical evidence suggest that use of PRP might be efficient in addressing surgical wound or even MRSA infections. In a rabbit model of MRSA osteomielitis, local application of PRP gel exterted antimicrobial activity even though not comparable with the Vancomycin control group [75]. Clinical application of PRP in treating high energy trauma soft tissue infected wounds was reported to induce healing [76]. Local application of autologous PRP in pressure ulcers in spinal injured patients reduced Staphylococcus aureus colonization [77]. To date there is no clinical evidence supporting the use of PRP as antimicrobial agents in orthopedic related infections as therapeutic agent or adjuvant therapy.
Role of PRP after muscle injury
The use of PRP in order to enhance recovery time and return to activity after muscle injury has become a relativelly common practice in sports medicine. Several preclinical studies demonstrate that PRP can increase skeletal muscle healing after acute injury. Local PRP administration increased expression of several myogenic factors at mRNA level acting on modlating the inflamatory response and myogenesis in the early stages after acute injury in rats [78]. A significant increase of the quantity of colagen was found in the PRP treated group compared to control at 7 days in a rat model of gastrocnemius injury, however morphological aspects of the msucle at 21 days was similar in the two groups [79]. A systematic review on articles reporting on preclinical and clinical results with the use of PRP until December 2012 for acute muscle injuries retrieved three in vivo animal studies and one human pilot study.
Pre clinical studies reported significant histological and accelerate muscle healing while in the clinical study athletes treated with repeated PRP injection were found to significantly faster than a retrospective control [80]. Higher level of evidence studies are beginning to accumulate in the recent years. A randomized controled trial on 75 patiens reported on effects of autologous PRP injections on time to return to play and recurrence rate after acute muscle injuries in recreational and competitive athletes. A single PRP injection significantly decreased the time of return to sports as well as pain severity score with no significant reduction of re-injury rates at 2 years follow up [81]. Current evidence supports PRP administration for accelerating muscle healing after sport related trauma while little is known about the effect on improving soft tissue healing in other traumatic contexts.
Conclusion
Increasing knowledge is accumulating about the intimate molecular mecanisms involved in tissue homeostasis, healing and functional recovery. The use of PRP as an autologus source of naturally occuring growth factors for accelerating reparatory processes is an appealing therapeutic strategy. As a versatile product of autologus origin that can be relativelly easy to obtain and to administrate intraoperatively or in outpatient settings, PRP used has spread consistently during recent years. Its use has proven to be safe with minimum complications for a large spectrum of applications in orthopedics and sports medicine. However, to this date, there are still a sum of scientific questions to be answered. Little is known about the exact GF content that can be obtained from a PRP sample. The particular modality of processing the blood sample , platellet enrichment and recovery, PRP storage or manipulation are likely to influence GF biodispoibility for a given therapeutic dose.
Moreover, a large individual variability can be expected to occur not only in the number of platellets that can be extracted but as well in the quantity and quality of GF that could have as result different proteomic profile of the samples. The development of cost efficient methods to assess PRP content and eventually the establishment of a biomarker based product characteristic requiered for every and each application will be likely to revolutionize the use of PRP in any field, includingly for musculoskeletal applications.
Current laboratory and preclinical studies are deepening knowledge about the mechanism and timing of GF involvmnet in specific patways during healing antiinflamatory processes. Setting up a cost efficient methodology of extracting a panel of growth factors from the PRP mixture has the potential to target a specific biological process more accurately. To date, the variability of administration (timing, preparation, doses) and large variations in assessing outcome results has made difficult to interpret the results from available clinical studies. High level evidence studies will be needed in order to enable the establishment of clear therapeutic indications eventually which product type would be more suitable for a given clinical situation.
To Know More
About Orthopedics and
Rheumatology Open Access Journal Please click on:
https://juniperpublishers.com/oroaj/index.php
To Know More About
Open Access Journals Please click on:
https://juniperpublishers.com/index.php



Comments
Post a Comment