Total Knee Arthroplasty Following Mycobacterium Kansasii Septic Arthritis in a Patient with Systemic Lupus Erythematosus and Essential Thrombocythemia - Juniper Publishers
Orthopedics and Rheumatology - Juniper Publishers
Abstract
Mycobacterium kansasii, like other non tuberculosis mycobacterial (NTM), is rare opportunistic infection that can lead to septic arthritis in immune suppressed patients. We report the case of a 56‐year‐old His panic female with systemic lupusery the matosus (SLE) and scleroderma on long‐term immune suppression, who developed an M.kansasii septic arthritis of the left knee in the setting of chronic lupus arthritis. Total knee arthroplasty (TKA) was delayed as the infection progressed into osteomyelitis of the distal left femur with an associated chronic, draining wound in the popliteal fosse. There are no reports in the literature documenting the treatment of this manifestation of M.kansasii in a patient with SLE, prior to TKA. Diagnosis requires a high index of suspicion, and was accomplished with synovial fluid culture and tissue biopsy. Treatment was accomplished with staged debridement and anti‐mycobacterial drug therapy along with tapering of immune suppressive regimen. Wound closure was achieved with a combination of a split thickness skin graft and negative pressure wound therapy (NPWT). Wound healing was augmented with hyperbaric oxygen therapy HBOT. A TKA was then performed, which yielded excellent results.
Keywords: Mycobacterium Kansasii; Chronic Wound; Knee; Total knee Arthroplasty
Abbreviations: SLE: Systemic Lupusery Thematosus; TKA: Total Knee Arthroplasty; NPWT: Negative Pressure Wound Therapy; AFB: Acid Fast Bacteria; ESR: Erythrocyte Sedimentation Rate; CRP: C‐Reactive Protein; OR: Operating Room; PICC: Peripherally Inserted Central Catheter
Introduction
Arthritis and arthralgias are noted in up to 95% of patients with systemic lupus erythematosus (SLE) but they are typically responsive to immune suppression and are often transient [1]. The arthritis of SLE is characterized by a non‐erosive inflammatory arthropathy (Jacouds Arthropathy); however, in the knee joint osteonecrosis can occur leading to accelerated articular degeneration. Studies show that patients with SLE require TKA at an average age of 56 compared to age 69 in matched controls, without auto immune disease [2].
The increased morbidity and mortality in SLE is well recognized and infection is a major cause of death, accounting for approximately 25% of deaths in a British cohort at a large tertiary care center [3]. In‐hospital post operative mortality for patients with SLE undergoing hip and knee arthroplasty was recently investigated using data from the Nationwide Inpatient Sample. The investigators found that patients with SLE had an odds ratio of 4.0(95%CI1.9‐8.0) for postoperative mortality with hip replacement and odds ratio of 1.2(95%CI0.2‐7.5) for mortality with knee replacements. In contrast, mortality risk for RA patients was similar to controls [4].
Non‐tuberculosis mycobacterial infection has been reported in patients with SLE [5], and typically presents later in the disease course and in patients receiving more potent immune suppression. M. kansasii is a typical mycobacterium that usually causes pulmonary infections, but has a predilection for the joints and soft tissue in patients with SLE [5,6]. It is particularly challenging to culture, and a prolonged period of time may pass before a definitive diagnosis is established, thus, leading to treatment delay and the potential for poor outcomes. Wound healing is known to be impacted by autoimmune diseases including lupus [7] and scleroderma [8].
Immunosuppressive therapies used to manage SLE can further impair immune defense and clearance of M. kansasii infections. The authors have obtained the patient’s informed written consent for print and electronic publication of the case report.
Case Studies
The patient is a 56‐year old female with long standing SLE overlap with scleroderma who had been taking chronic prednisone for management of interstitial lung disease and recurrent pericarditis. She initially presented with chronic recurrent left knee effusion at an outside hospital.
Synovial fluid analysis was initially benign, with a white cell count of 2100 and no crystals present. Anti‐inflammatory agents and a trial of intra‐articular corticosteroid injections failed to relieve her pain , and the patient was referred for an exploratory arthroscopy. Post‐operative analysis of the synovial fluid revealed 56,000 white cells and synovial cultures were positive for acid‐fast bacilli (AFB) after 10 days of incubation. It was believed at the time to be an external contaminant. Without a definitive pathogen, the patient was treated empirically with a four‐week course of vancomycin and piperacillin‐tazobactam.
Her pain slightly improved and she continued to have difficulties with ambulation. One year later, she developed a new, painful swelling in her left popliteal fossa. An MRI was suggestive of a Baker’s cyst; however, no fluid could be a spiraled by interventional radiology. An aggressive attempt at arthroscopic synovectomy and closure of the popliteal wound then followed.
The wound broke down two days later. Four further attempts at surgical closure of the wound resulted in dehiscence. One‐year after initial presentation, the surgical wound had transformed into a recalcitrant, painful, non‐healing fistula a with malodorous, grey drainage. The patient was reporting fevers, fatigue, and an eight-pound weight loss. No definitive pathogen had been cultured.
At the time of presentation, the patient’s medications included azathioprine 150 mg daily, cholecalciferol 5000 units once a day, calcium citrate‐vitamin D 1000 units daily, aspirin 81 mg once daily, acetaminophen 500 mg daily, dexlansoprazole 60 mg once daily, prednisone 4.5 mg daily, zoledronic acid 5mg yearly infusions, topical diclofenac sodium gel, and tramadol 50 mg every six hours.
On examination, vital signs, cardiac and pulmonary exams were within normal limits. The patient had a swollen left knee that was red and warm to the touch. There was an open, exudative, mildly malodorous, 1.5 x 0.4 x 2.5 cm wound in the left popliteal fosse with surrounding erythema. Edema was 1+, extending downward into left lower limb. Dorsalis pedis and posterior tibial pulses were 2+. No neurological deficits were appreciated.
Laboratory studies revealed mild normocytic anemia with a hemoglobin count of 8.1 g/dL, an elevated white cell count of 27.4 x 103 uL, a creatinine 0.52 mg/dl, and an albumin of 2.6. Transaminases were within normal limits. Her erythrocyte sedimentation rate (ESR) was 98 mm/hr and the serum Creactive protein (CRP) level recorded as 15.9 mg/L. Blood, urine and stool cultures were negative.
Management and Outcome
Our initial impression was that she suffered from septic left knee arthritis, likely related to a history of SLE and immune suppression. We were concerned that the popliteal wound tracked into the joint. A multidisciplinary team of infectious disease, orthopedics, rheumatology and plastic surgery proceeded with a management strategy beginning with staged debridement and serial cultures to determine a pathogen for targeted antibiotic therapy.
The patient would then be closed surgically with the aid of a graft or soft tissue reconstruction. TKA would follow after stabilization of the soft tissue. Optimization of her SLE medication regimen was paramount for successful management at all stages.
The patient under went three excision a debridement and final closure of the wound. Multiple, purulent deep tissue abscesses were identified within the wound in popliteal fosse, and were incised and drained. The wound bed was derided down to healthy appearing tissue, leaving a 9 x 3 cm defect. Copious irrigation prepared the wound for placement of a NPWT device. Cultures of the wound were obtained before and after debridement to determine organism speciation, sensitivities, and assess for clean margins. Cultures from the first two debridements were positive for methicillin resistant’s resistant staphylococcus aureus (MRSA) but not AFB.
The infectious disease team was consulted and the patient was place on intravenous vancomycin and cefepime. Three days later the patient was taken to the OR for a final debridement and closure with a split thickness skin graft. A peripherally inserted central catheter (PICC) line was placed and the patient was discharged one day later to receive daptomycin 300mg IV daily for 30days. Azathioprine and prednisone were continued for management of SLE, per rheumatology. Post‐debridement cultures including AF yielded no growth.
She required another “take‐back” one month later due to poor graft adherence and renewed drainage. Cultures remained negative.
After 5 months of follow up and difficulty healing, the patient was reevaluated for new drainage from the wound. An MRI of the left lower limb was concerning for osteomyelitis in the distal femur. The patient was taken back to the OR for debridement and culture of the involved tissues. Methylene blue was injected to identify the joint‐space during the surgical approach. A posterior knee incision was made into the popliteal fosse, and multiple specimens, including the capsule, were sent for culture and pathology. 3 x 2 cm, cheesy, caseating mass, adjacent to the left medial femoral condyle was evacuated (Figure 1).
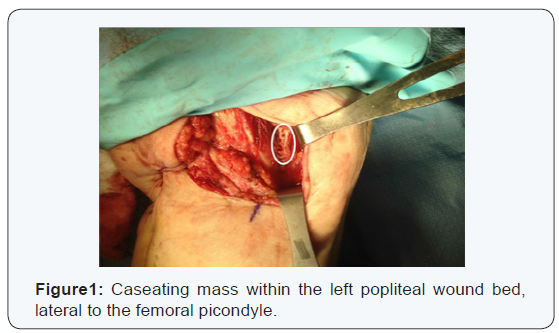
Two draining sinuses within the 10 x 2 cm wound bed were debrided with sharp curettage. The gram stain was unhelpful and the cultures again returned with no growth on AFB media. The patient was sent home on moxifloxacin 400 mg PO daily and instructed on daily dressing changes with gauze dressings.
She presented one month later with a fever, and was reevaluated to for the source of this indolent and here to fore unexplained infection. There was a suspicion that mycobacterium could be involved because mycobacterium species are known to cause a tenosynovitis and persistent wounds, with cultures often being negative. This hypothesis was supported, by the caseous nature of the drainage, the history of chronic immunosuppressive therapy, and the priorpositive AFB culture from the outside hospital. Mycobacterial infection had long been suspected but without subsequent cultures, a definitive diagnosis could not be made. A decision was made to discontinue azathioprine, and the patient underwent subsequent evacuation of there‐accumulated as eating mass from the joint space in the left knee. AFB cultures were sent again; this time the cultures returned positive for M.Kansasii, and the patient was started on a triple therapy with rifampin 150mg BID, isoniazid 300mg daily, and azithromycin 500mg daily. NPWT was maintained after discharge.
She presented one month later with a fever, and was reevaluated for the source of this indolent and heretofore unexplained infection. There was a suspicion that mycobacterium could be involved because mycobacterium species are known to cause a tenosynovitis and persistent wounds, with cultures often being negative. This hypothesis was supported, by the caseous nature of the drainage, the history of chronic immunosuppressive therapy, and the prior positive AFB culture from the outside hospital. Mycobacterial infection had long been suspected but without subsequent cultures, a definitive diagnosis could not be made. A decision was made to discontinue azathioprine, and the patient underwent subsequent evacuation of the re-accumulated caseating mass from the joint space in the left knee. AFB cultures were sent again; this time the cultures returned positive for M. kansasii, and the patient was started on a triple therapy with rifampin 150mg BID, isoniazid 300mg daily, and azithromycin 500mg daily. NPWT was maintained after discharge.
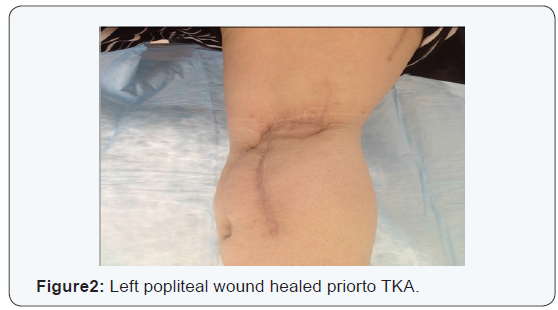
The antimycobacterial drugs were discontinued two weeks later due to a drug reaction, which manifested as a severe pruritic rashm flu-like symptoms, and fatigue. Four months later, a sinus within the wound re-opened and was treated with iodosorb and acetic acid dressings. AFB cultures at this time were negative. The patient was referred to hyperbaric medicine team for hyperbaric oxygen therapy (HBOT). She underwent a total of 30 dives over the course of 45 days, at 2.0 atmospheres for 90 minutes each. Three months later, the left posterior knee wound had completely healed and the patient was ambulating well with the aid of a knee brace and a cane (Figure 2).
She was restarted on antimycobacterial therapy, with a new regimen of rifampicin 300 BID and ethambutol 400 mg TID.
She remained on ethambutol for 25 month until it was discontinued due to ocular findings, one month prior to TKA. Almost four years after the initial presentation, the patient successfully underwent TKA. Following aggressive physical therapy she has full range of motion of the knee and is ambulating without significant pain. Rifampin was continued perioperatively, and discontinued six months after TKA, after a total of 33 months of treatment.
Discussion
This case demonstrates the challenging diagnosis and treatment of M. kansasii septic arthritis, osteomyelitis, and a fistulous chronic wound in a patient with SLE. There should be a high degree of suspicion for atypical mycobacterial disease in patients presenting with persistent septic arthritis with autoimmune disease, and who are significantly immunosuppressed [9].
A study by Mok et al analyzed a cohort of 725 SLE patients with previous NTM infections and found that compared to mycobacterium tuberculosis infections, NTM infections such as M. kansasii, were more likely to present in extra pulmonary sites (P=0.006), in those with a longer duration of SLE (P<0.001), and in those with a cummulative higher dose of corticosteroids (P=0.01)[5].
M. kansasii infections typically involve soft tissues tissues, bones, bursae, tendon sheaths and large or medium joints [10]. Patient with SLE are especially predisposed to extrapulmonaryinvolvement [11]. Seeding of deep tissues by ,M. kansasii?, can occur via hematogenous spread, but is more frequently caused by trauma and contaminated medical instrumentation or solutions, such as local anesthetics and steroids [10,12,13].
Tap water is believed to be a major reservoir and source of contamination for M. kansasii [13,14] Diagnosis of M. kansasii septic arthritis is made with culture of synovial fluid or biopsy of the synovium; however, cultures are often negative. This may be due to poor inoculums yielded from synovial fluid or the fact that M. kansasii is slow growing and fastidious in vitro[11]. An abundance of neutrophils are often the only findings on diagnostic work up of affected tissues, and thus diagnosis can be difficult [14].
Corticosteroids and immunosuppressive agents, which are commonly used to manage SLE, can mask the inflammatory response, which may further delay diagnosis. There are documented reports of patient with destructive arthritis of unknown origin persisting for 11 years after presentation, until the infection converted to a granulomatous lesion, from which positive cultures were ultimately obtained [11].
Combination pharmacotherapy is the cornerstone of treatment of mycobacterial infections and monotherapy is not recommended due to the potential for development of drug resistance [15] M. kansasii is usually susceptible to isoniazid, ethambutol, rifampin, ethionamide, streptomycin, and clarithromycin. Combining rifampin specifically with the other drugs significantly improves the potency of the regimen, and lowers rates of resistance and subsequent relapse [14].
The recommended treatment for patients with rifampin susceptible M. kansasii is a daily regime of rifampin 10 mg/kg/ day (maximum 600 mg), ethambutol 15 mg/kg/day, isoniazid 5 mg/kg/day (maximum 300 mg), and pyridoxine 50 mg/day until 12months of negative cultures are obtained. Rifampin resistant M. kansasii should be treated with a three‐drug regimen consisting of clarithromycin or azithromycin, moxifloxacin, ethambutol, sulfamethoxazole, or streptomycin as the susceptibility data allows [14].
Often times, the microbiology data in the immunocompromised host can be unclear, with multiple microbes contributing to the overall clinical presentation. Bacteria can form dense colonies within bio film that can subvert host defenses and antibiotic drugs. Further, corticosteroids used to treat SLE, aside from impairing wound healing [16] and systemic response to infection, have been found alter bio film formation in animal models. While the total production of biofilm can be reduced with corticosteroid use, such therapy may alter the biofilm in such a way that resistance of bacteria to to certain antibiotics is enhanced [17]. As a result, patients with deep tissue involvement should be managed pharmacologically and surgically, with aggressive antimicobacterial therapy, staged debridement, and wash out of all compromised tissues [17-20].
Conclusion
In conclusion, although rare, M. kansasii should be added to the differential diagnosis in patients with SLE and other immune diseases, and persistent septic monoarthritis. This diagnosis should especially be considered in patients receiving chronic immunosuppressive therapies. Since immune diseases are known to impact wound healing, and immune suppression can contribute to impaired host defenses, it is crucial to both optimize immune therapy and aggressively treat the infected joint. A multidisciplinary approach to the care of patients with autoimmune disease and septic arthritis can be used to minimize risks and improve outcomes in this patient population.
To Know More About Orthopedics and Rheumatology Open Access Journal Please click on: https://juniperpublishers.com/oroaj/index.php
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/index.php
For more about Juniper Publishers Please click on: https://juniperpublishers0.wixsite.com/juniperpublishers



Comments
Post a Comment